Existing widespread use in China
RLRL therapy’s efficacy and safety has been validated through a comprehensive ongoing clinical trial program. The results, published in leading peer-reviewed scientific publications show that controlled exposure to the Eyerising Myopia Management Device RLRL therapy can control the progression of myopia. By improving the metabolic rate and blood flow of the surrounding structure of the eye, we can strengthen it and control the growth of the eye’s axial length.
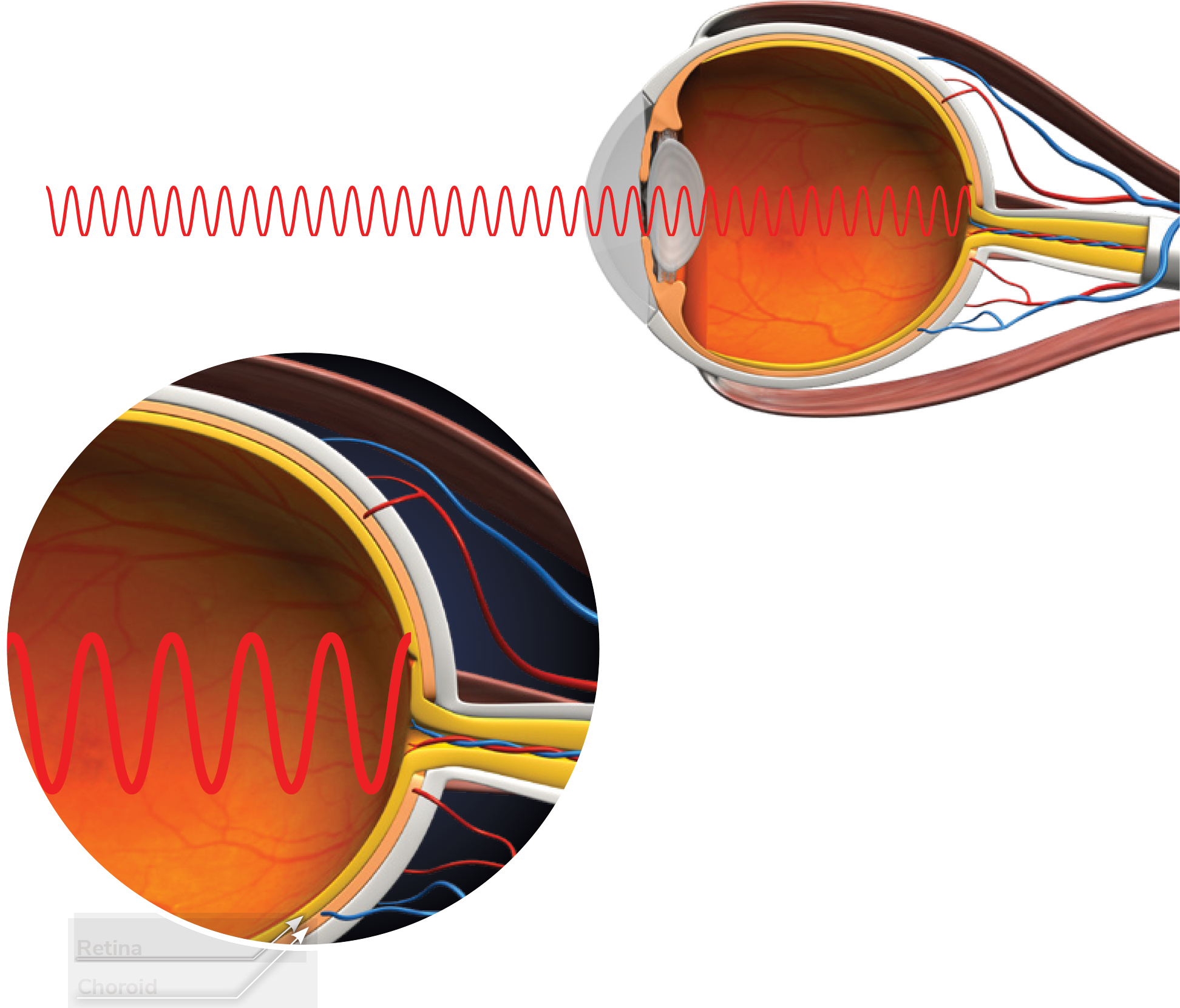
How the Eyerising Myopia Management Device works
In a normal eye, light passes through the lens with the focal point correctly reaching the retina.
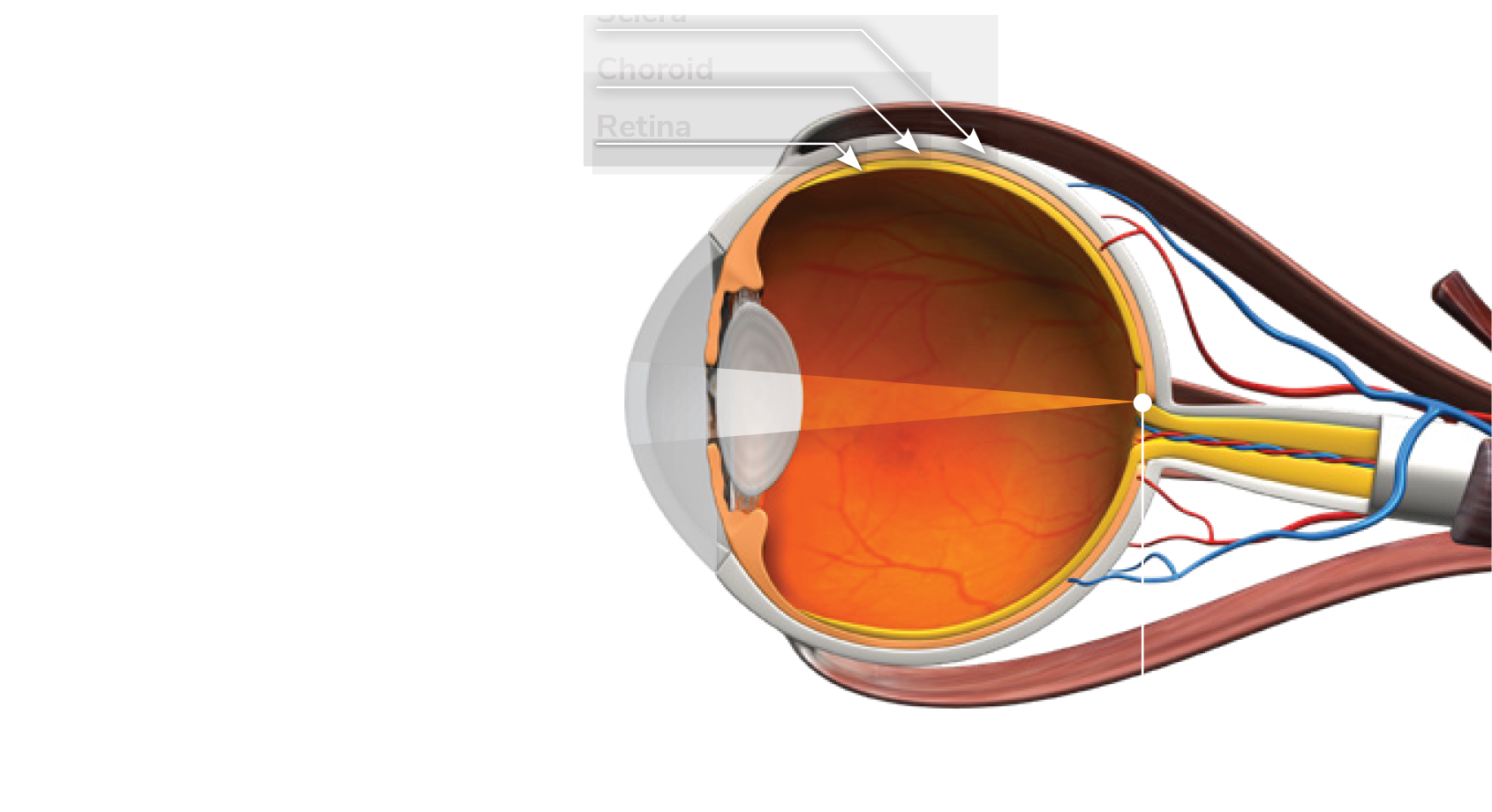
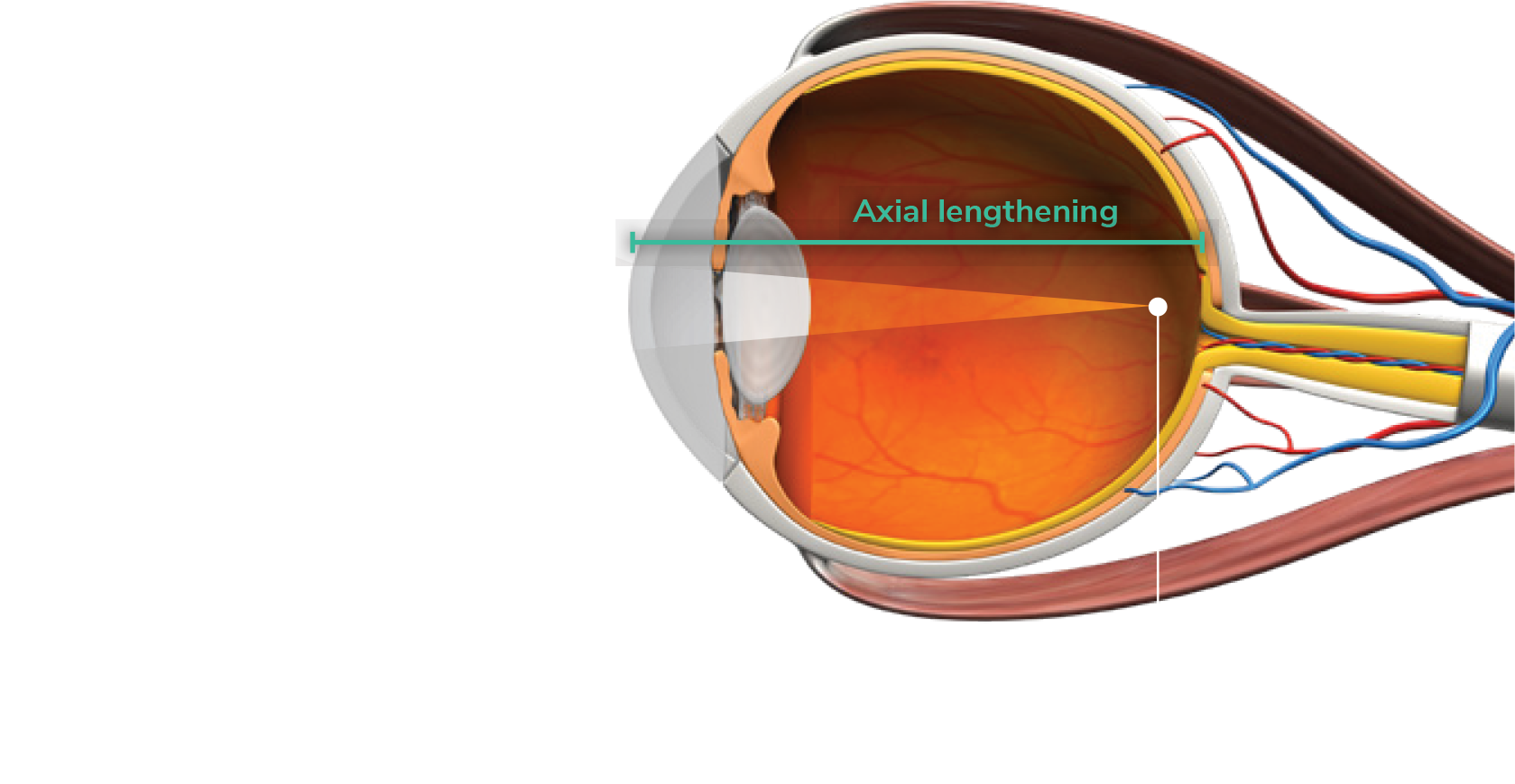
As myopia progresses, axial lengthening and focal point mis-alignment increase. Continued axial lengthening and focal point misalignment leads to high myopia.
The device delivers RLRL therapy to the retina.
Blood flow is stimulated to help slow the axial length’s further elongation and control myopia’s progression.
The device delivers Repeated Low-Level Red-Light therapy to the ocular fundus.
Blood flow is stimulated and increases to rethicken the choroid layer to help slow the axial length’s further elongation and control myopia’s progression.
See reference (1)
See reference (2)
Extensive clinical trial program
An extensive clinical trial program has established the impressive effectiveness and safety of using Repeated Low-Level Red-Light (RLRL) therapy and the Eyerising Myopia Management Device for the treatment of myopia. In addition to the six peer-reviewed clinical papers located in the Science Hub, there are currently multi-ethnic clinical trials underway in Australia and the USA and high and adult myopia clinical trials underway in Japan.
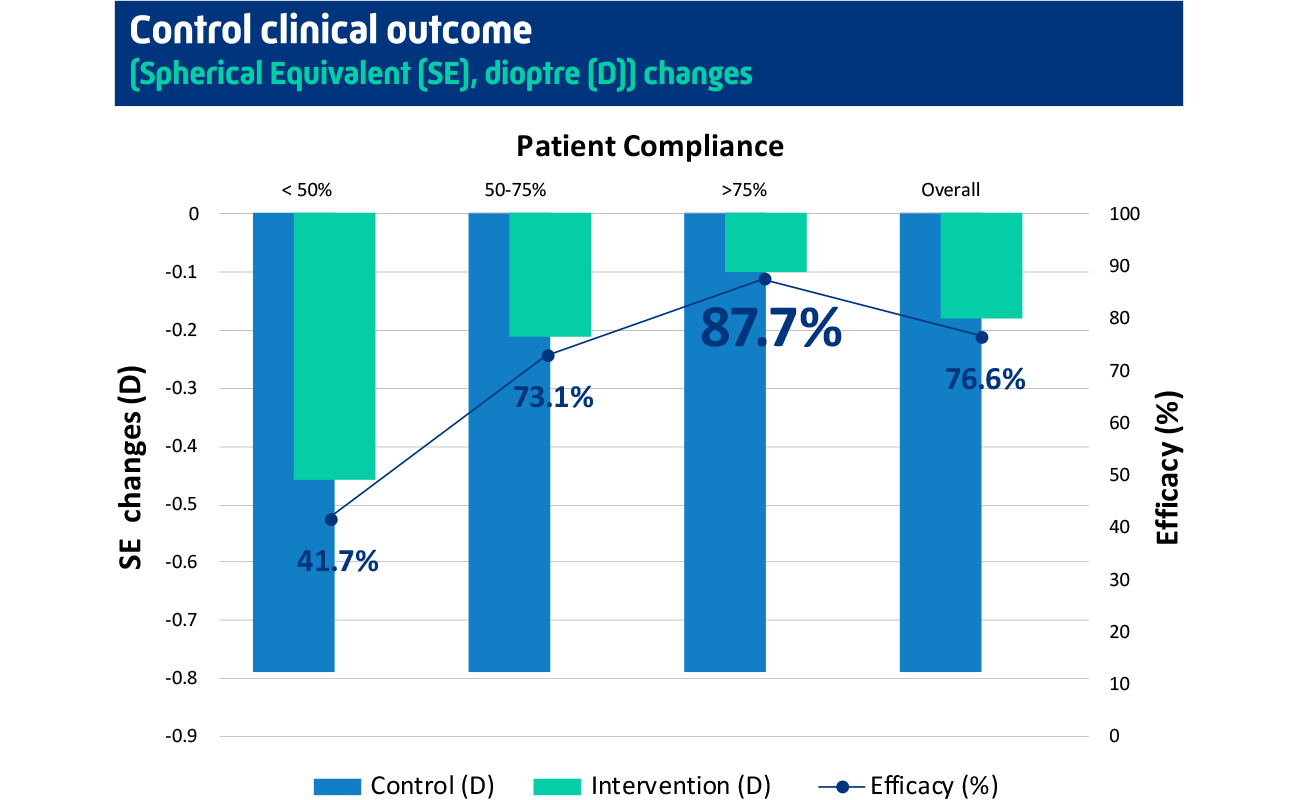
Achieving up to 87.7% myopia control efficacy when patient has >75% compliance¹
Overall, children in the RLRL-plus-spectacles group had a 76.6% reduction in myopia progression compared with children in the single vision spectacles-only group (SVS). There was an 87.7% reduction in children who had high compliance with the treatment schedule (>75%). There were no known significant side effects, and the overall, the treatment was well tolerated by the children participating in the trial.
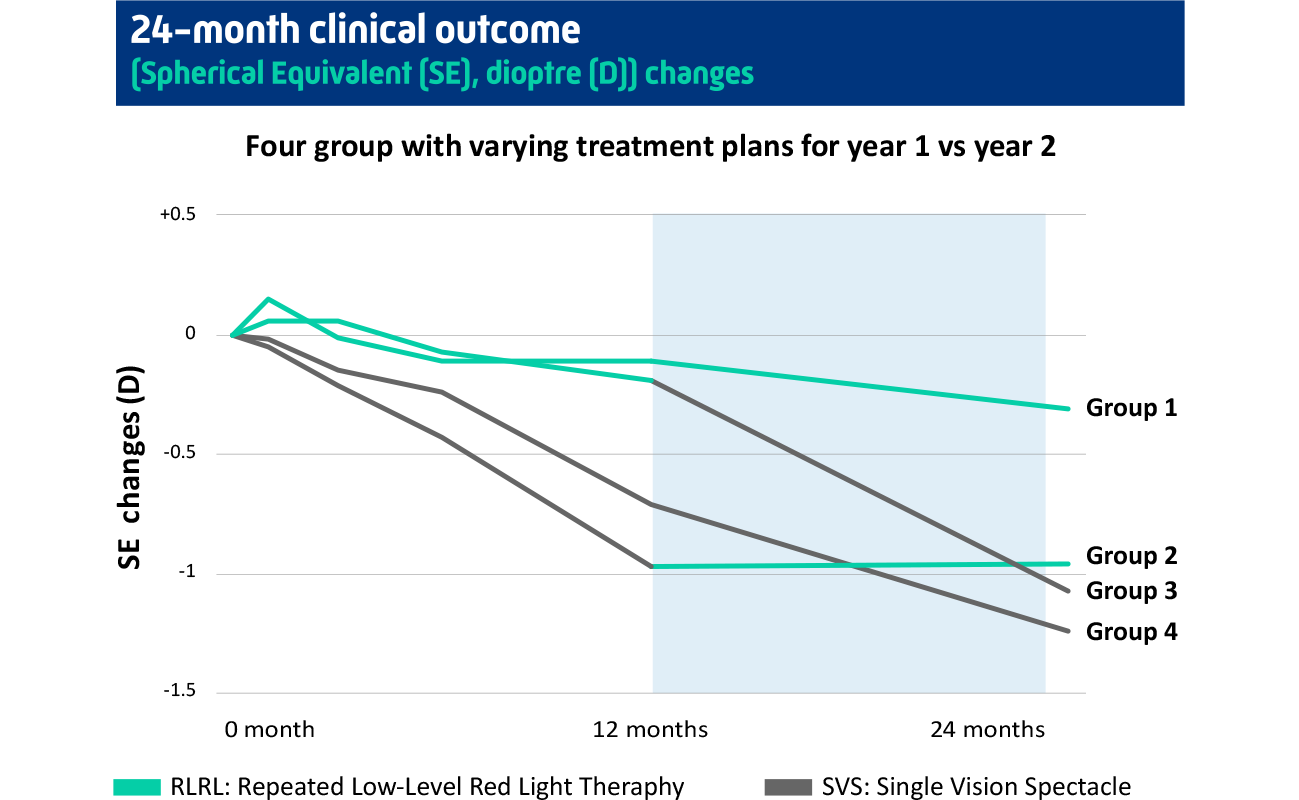
At the end of this study, the participants were invited to enrol in a follow-up study for a further 12 months.2 Those children who continued with RLRL therapy had a 75% reduction in myopia progression, and those who switched to RLRL therapy in the second year had a 31% reduction in myopia progression when compared with those children who stayed with SVS. Visual acuity remained the same with no known side effects or severe adverse events.
View our most important scientific papers
A 12-month clinical trial conducted on 264 participants published on Ophthalmology
24-month follow-up clinical trial conducted on 199 participants published on CEO
Clinical trial to assess the efficacy and safety of treatment in controlling myopia progression compared to a sham device with only 10% of the original power published on Ophthalmology
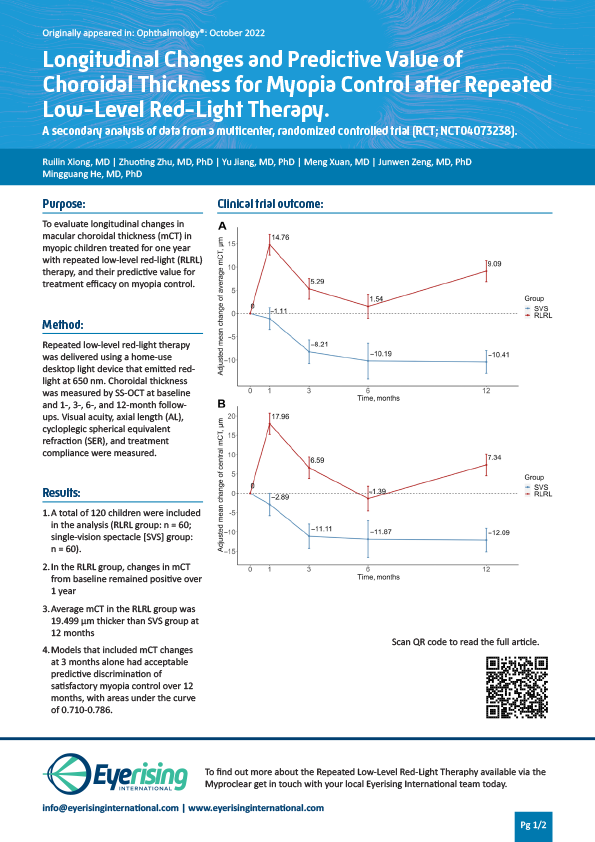
A multicenter, randomized controlled trial (RCT) to evaluate longitudinal changes in macular choroidal thickness in myopic children treated for one year with the treatment published on Ophthalmology
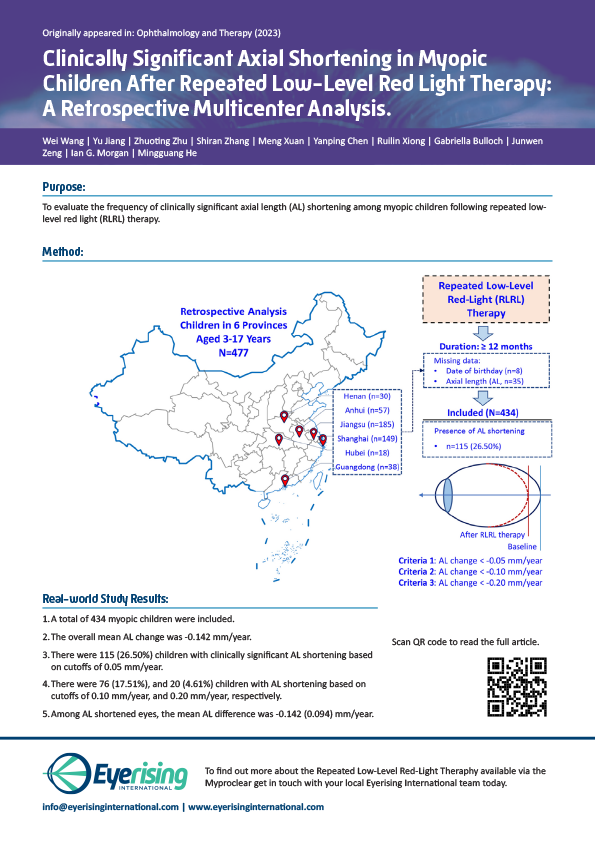
Clinically Significant Axial Shortening in Myopic after RLRL
Axial Shortening in Myopic Children after Repeated Low-Level Red-Light Therapy
References
- Jiang Y, Zhu Z, Tan X et al. Effect of Repeated Low-Level Red-Light therapy for myopia control in children. Ophthalmology 2022; 129 (5): 509–519.
- Xiong R, Zhu Z, Jiang Y et al. Sustained and rebound effect of Repeated Low-Level Red-Light therapy on myopia control: a 2-year post-trial follow-up study. Clinical & Experimental Ophthalmology 2022 (August); published online ahead of print. 3. Dong J, Zhu Z, Xu H et al. Myopia control effect of Repeated Low-Level Red-Light therapy in Chinese children: a randomized, double-blind, controlled clinical trial. Ophthalmology 2022 (August); published online ahead of print.
- Compliance report IEC 60601-1-2:2014
- CFDA website: https://www.nmpa.gov.cn/
- Xiong, Ruilin, et al. “Longitudinal changes and predictive value of choroidal thickness for myopia control following Repeated Low-Level Red-Light therapy.” Ophthalmology (2022).
- Dong, Jing, et al. “Myopia Control Effect of Repeated Low-Level Red-Light Therapy in Chinese Children: A Randomized, Double-Blind, Controlled Clinical Trial.” Ophthalmology (2022).